A critical early decision is determining what aspect of manufacturing flexibility is most important before initiating facility design. Surveys we have taken on this topic indicated a clear correlation between an individual’s job responsibilities and how they prioritize flexibility goals. Respondents tended to value that aspect of flexibility that is most useful in their role. Acknowledging that this is a natural human inclination, the survey findings underscore the importance of clearly defining project goals from the onset and consistently reinforcing them with the project team throughout execution.
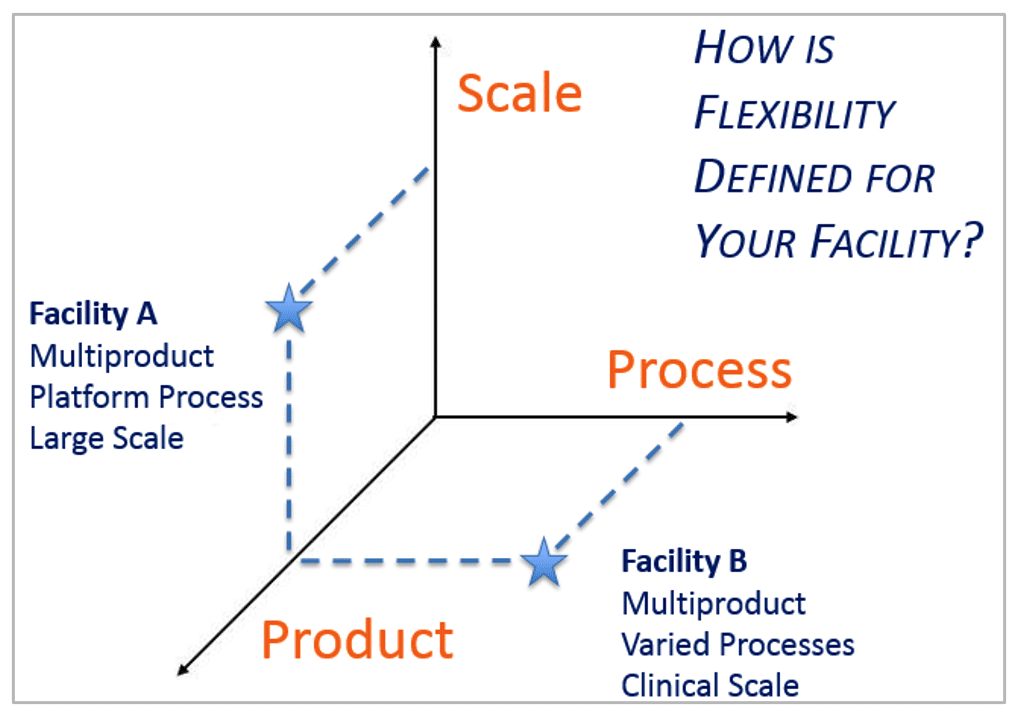
This led to thinking about the dimensions of flexibility within a GMP facility, and how flexible design solutions differ depending upon the manufacturing mission of the facility.
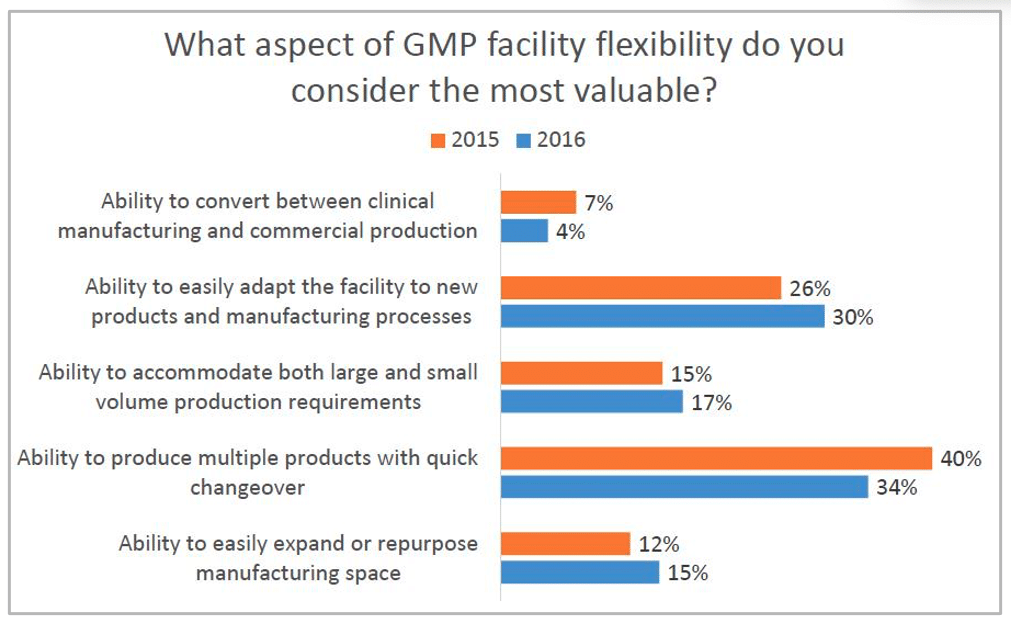
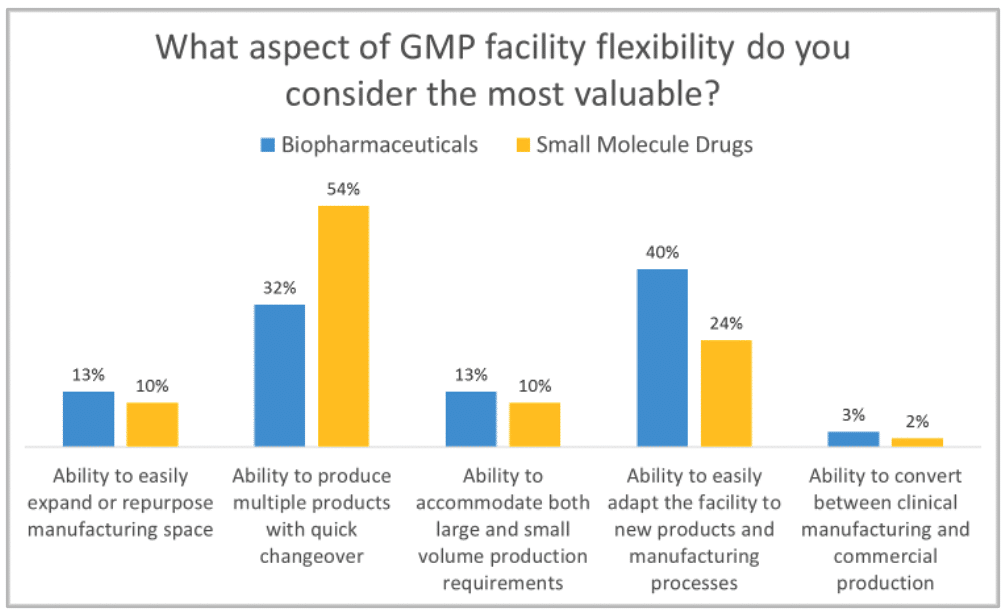
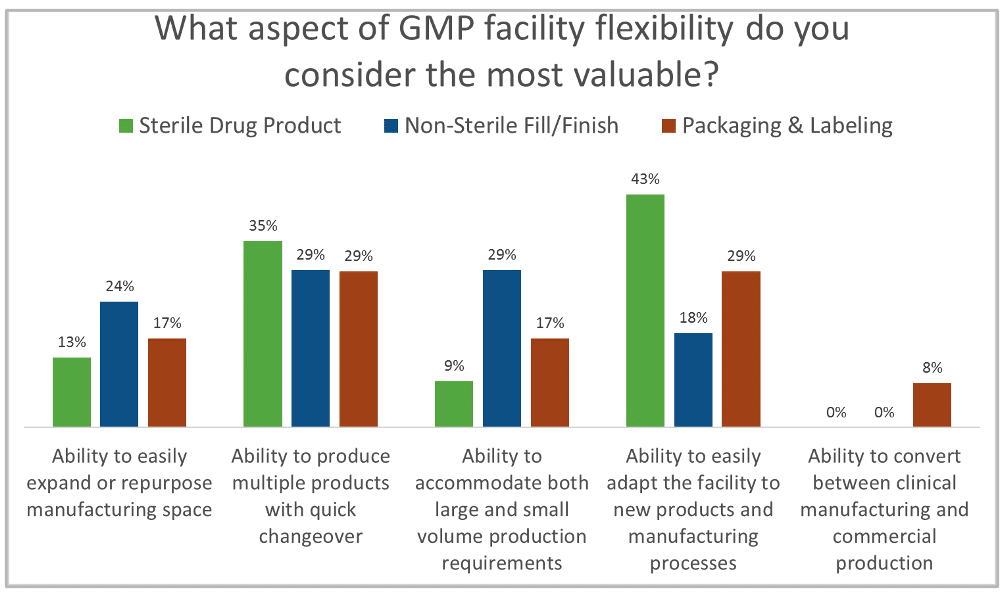
As part of Facility Focus, an industry-wide survey of the life sciences industry conducted by Carroll Daniel Engineering, in partnership with INTERPHEX, industry insiders – those in engineering and operations roles – were asked what aspect of facility flexibility is most valuable. Survey results were consistent year after year: respondents most highly value the ability to produce multiple products with quick changeover, followed by the ability to easily adapt the facility to new products and manufacturing processes. The strongest preference was expressed by those working in drug substance facilities.
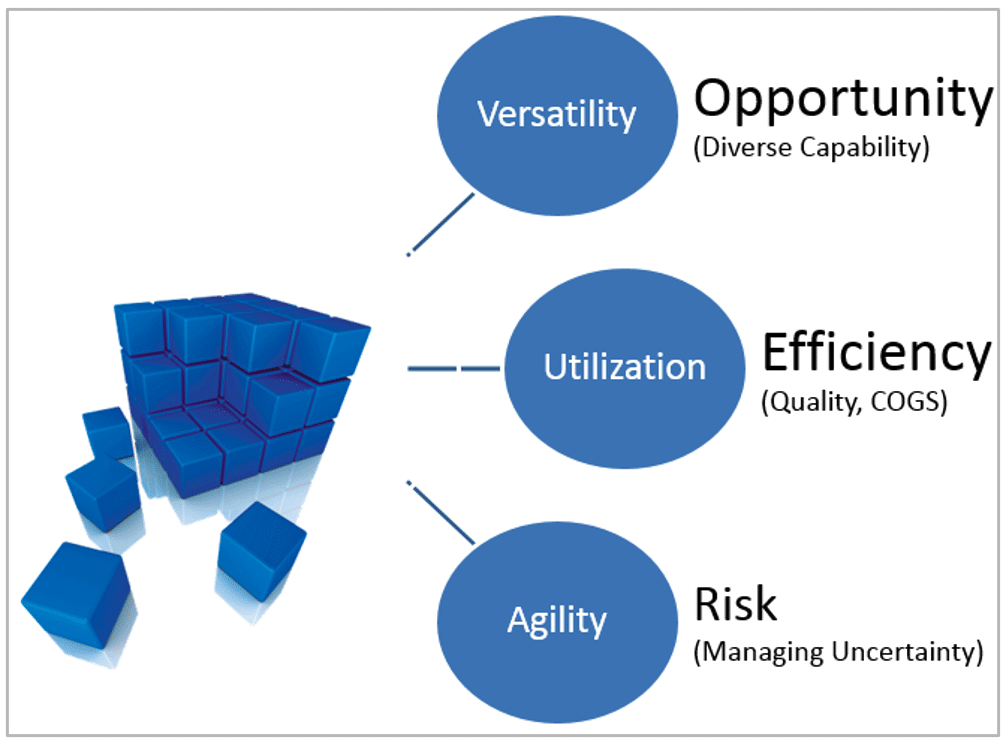
For GMP Facilities, Flexibility → Opportunity + Efficiency + Risk Mitigation